At OcyonBio, we understand the importance of having a reliable, cost-effective solution for manufacturing and developing biologics, gene and cell therapies, and other therapeutics. That is why we have designed a PDMO model that provides the same functionality as a CDMO, with added flexibility for controlling manufacturing and improving the speed-to-market.

We are dedicated to ensuring that our partners can succeed in their endeavors with the support of our experienced team of professionals ready to help you navigate the complexities of drug development and production.
Our facility is growing rapidly, covering two buildings with over 200,000 sq ft of space and room to grow to over 1 million sq ft. The developing campus location is ideal for pharmaceutical activities and research due to Puerto Rico’s advantageous tax incentives, proximity to the U.S., and established infrastructure that has supported over 60 years of pharmaceutical presence on the island.
Just a Quick Note:
InnovationsOfTheWorld.com has partnered with Trade License Zone (TLZ) to support global innovators looking to expand internationally. Take advantage of the UAE’s Free Zones—enjoy streamlined setup, low corporate taxes, and a strategic gateway to the Middle East and beyond.
Get Your UAE Free Zone License Fast & Easy!
Our innovative PDMO model approach features an array of critical advantages:
Cost reduction – Our location, grants, credits, and decree can get you up to 50% of all your annual cash rebate.
Increased speed – We have ready ISO 8/7 space to support clinical and commercial manufacturing that can be customized in months from our ‘white box’ stage.
Build CMC – We have a team of experts in CMC and Regulatory that can generate all packages required for the U.S. and E.U. submissions.
Maximized efficiency – Our infrastructure allows for cGMP in the box so the company can focus on science while we focus on phased appropriate regulatory compliance.
Take control – Our spaces can be leased, giving complete control of space, equipment, schedule, and resources.
Relocate to Puerto Rico – In Puerto Rico, USA; we speak three languages, English, Spanish, and cGMP (Puerto Rico produces the top 10 biologics in the world).
Take advantage of World Class Campus – Our campus houses multiple capabilities where the company team can leverage our ecosystem from blood processing to fill and finish of the drug product.

Leverage our expertise – Our team has expertise in cGMP, Regulatory compliance, CMC, and phased-appropriate facility design and operations.
Utilize our Quality Systems – Our electronic paperless systems include QMS, LIMS, MES, and SAP to support data collection, reporting and secure cloud-based access for 100% data available anywhere in the world.
Benefit from our Ecosystem – Our facility can produce and test blood-derived cells, AAV, Lenti, & Retroviral Vectors Gene and Cell therapy, biologics, fill-finish, Quality Control Lab, and final packaging to comply with complete commercialization requirements reducing the need for external partners.
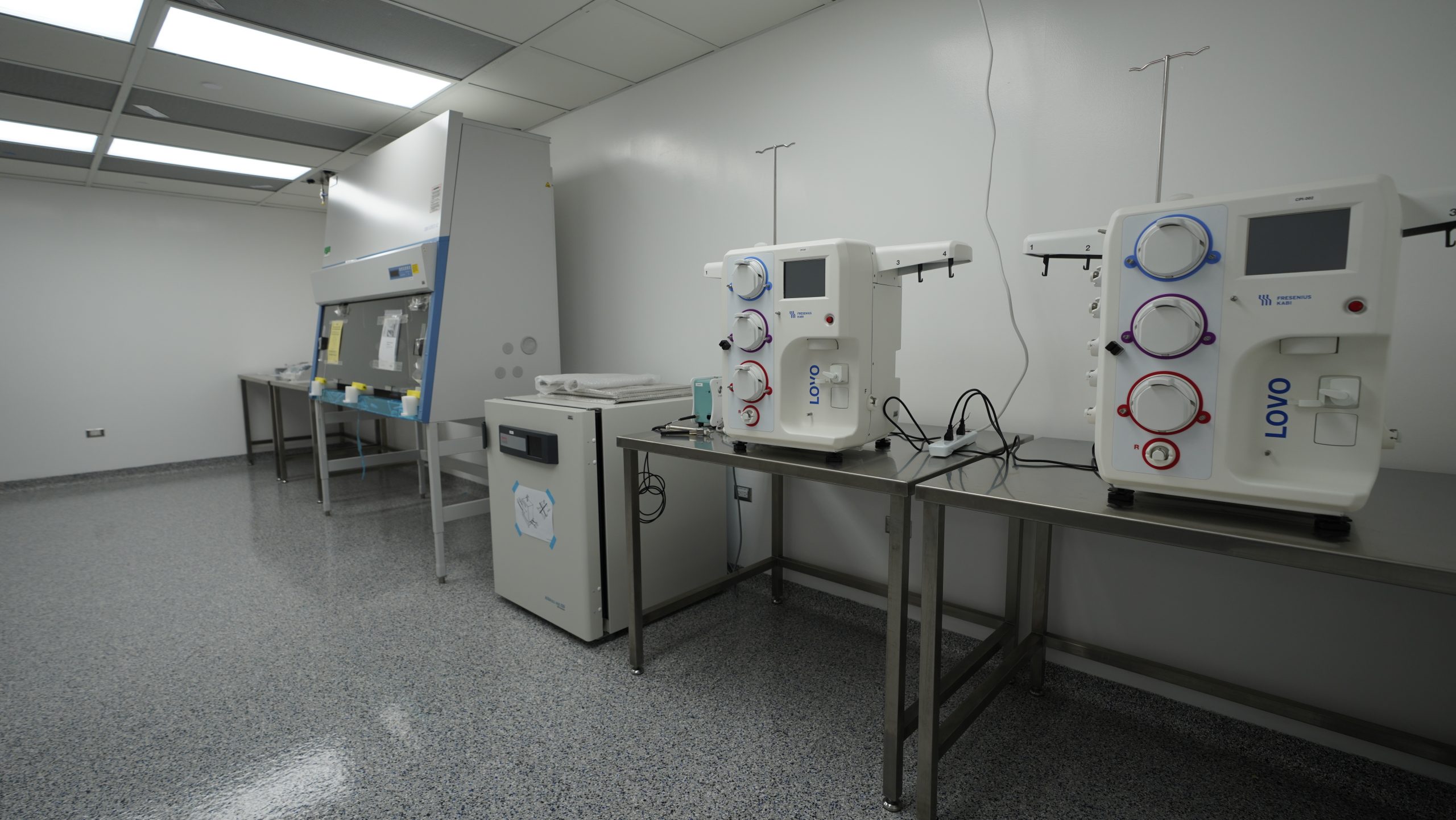
Furthermore, the current industry environment presents several challenges for novel drug developers. Our team is actively working to identify and address these challenges to ensure that our partners have a successful journey from concept to commercialization. To that end, we are focusing on providing innovative solutions that address the specific needs of each of our clients and enable them to succeed in the current industry environment.

The first challenge, an unprecedented demand in the Cell, Gene, Biologics, and Viral manufacturing CAPACITY due to a combination of the continuing covid-19 vaccine needs and the growing market. This has created an immense strain on capacity and resources. Our campus will add to global capacity by offering clean room spaces ranging between 200-50,000 sq ft.(with expansion projections for increasing capacity) for R&D to commercial manufacturing.
The second challenge, acquiring and retaining TALENT with adequate experience aligned with an ideal candidate profile. Tapping into a talented workforce of 90,000 manufacturing employees. Of that, tens of thousands specialize in pharma production. OcyonBio has put in place a talent database to support immediate hiring and onboarding managed with our customized training system. Our pre-screened database of talent will help client hiring plans through 2026, drawing from the 25,000 majors in
STEM that graduate every year locally and many Puerto Ricans leading their professions on the mainland. We have already begun the process of recruiting highly qualified professionals and are in the process of hiring a team of local experts to ensure that we can best serve our customers and patients.

The third challenge, SPEED to market, is impacted by the lack of space and long wait times for manufacturing slots in traditional CDMOs. We provide a wide range of ready-to-go clinical ISO7/8 clean rooms, which are customizable within 60-180 days. We provide our partners with production autonomy, including complete schedule control.
The fourth challenge, high COSTS of establishing production. Puerto Rico’s businesses & citizens have all the legal rights afforded by the U.S. Constitution, whilst having several unique advantages, including operating within a jurisdiction. One of these advantages is the non-dilutive funding of up to 60% of the total project cost, with incentive programs being leveraged by existing pharmaceutical companies. Additionally, local loans are available to reduce the impact on cash flows.

The development of the OcyonBio Campus will not only solve many industry challenges, but it will also create jobs and attract businesses and entrepreneurs to Puerto Rico. It will also provide the necessary infrastructure and resources to develop new therapies, treatments, and drugs to address global health needs. The facility has advanced research and development capabilities, including advanced analytical instruments, sophisticated laboratories, and state-of-the-art manufacturing equipment. This expansion is the latest in a series of investments we have made in Puerto Rico, and we are excited to be a part of the island’s economic development. As we continue to grow, we look forward to further strengthening our presence in Puerto Rico and playing an important role in the local community















