The clinical research industry continues to evolve and improve, seeking ways to boost participant engagement and facilitate more active, remote data collection. These explorations lead Biopharma and CRO customers toward decentralized study approaches, which are designed to increase operational efficiency and participant convenience. A decentralized study is conducted primarily in a participant’s
Leading the way
THREAD’s innovations in technology and approach are the result of continued collaborations with sites, participants, regulatory agencies, sponsors and CROs. Through this perspective and experience they solve some of the toughest industry challenges in clinical research study designs and methods.
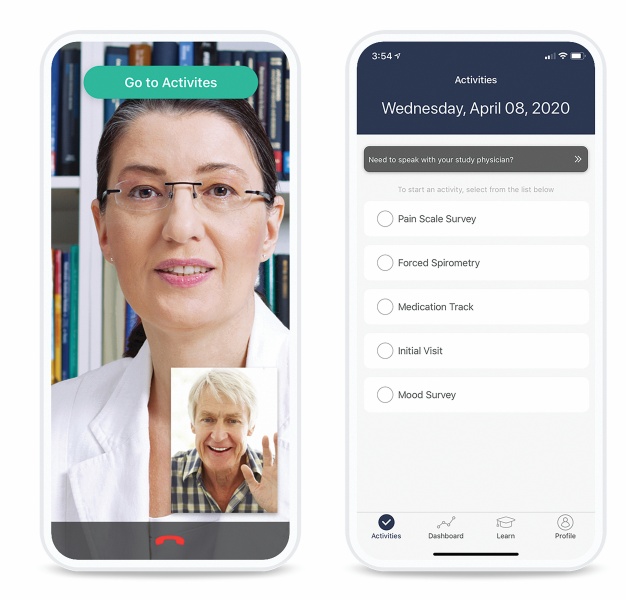
Sponsors and CROs can remotely capture data from participants and sites during, in between and in lieu of in-clinic visits – all securely through one unified platform. These solutions are rapidly enrolling hundreds of thousands of participants in studies across the globe, covering a variety of therapeutic areas.
Just a Quick Note:
InnovationsOfTheWorld.com has partnered with Trade License Zone (TLZ) to support global innovators looking to expand internationally. Take advantage of the UAE’s Free Zones—enjoy streamlined setup, low corporate taxes, and a strategic gateway to the Middle East and beyond.
Get Your UAE Free Zone License Fast & Easy!Being a leader in innovation requires the ability to adapt quickly to a changing environment. As the world braced for impact during the COVID-19 pandemic, THREAD responded to the need for clinical trial risk mitigation tools and launched THREAD Express. By using THREAD Express, customers deploy solutions that are intended to be aligned with guidance provided by regulators, including the U.S. Food and Drug Administration and the European Medicines Agency, to help manage clinical trials during the COVID-19 pandemic.
Power through innovation
THREAD’s unified platform provides user-friendly experiences and advanced capabilities. It is a technical technology solution that pulls all facets of clinical study design together – without the strain of managing multiple partners. Sponsors and CROs have an easy-to-use system that delivers flexibility, manageability and scalability while maintaining security and speed.
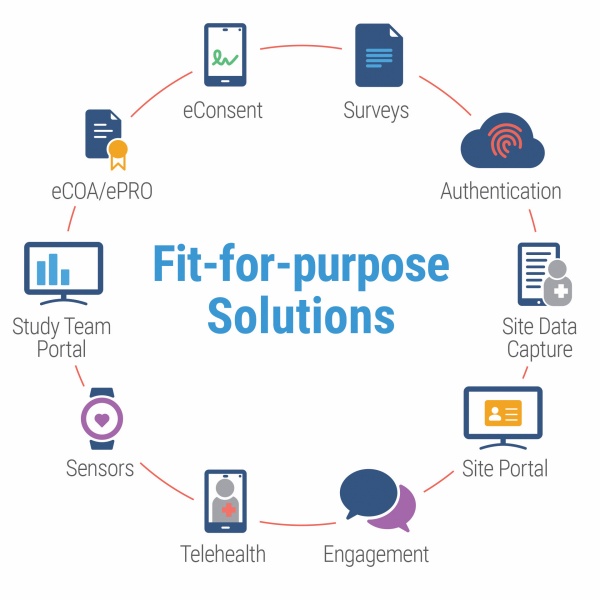
This secure, cloud-based platform provides a self-guiding configurator for easy study start-up. A full suite of fit-for-purpose solutions are available to support the needs of each individual study, including: eConsent, eCOA/ePRO, surveys, study team portal, site data capture, authentication, telehealth, sensors, site portals and participant engagement tools.

Turnkey – press launch
If it’s service you need, initiate the THREAD LaunchKit. Sponsors and CROs engage THREAD’s experts from kick-off meeting to live platform. The LaunchKit is a proven process to help design, configure, deploy and manage decentralized studies.
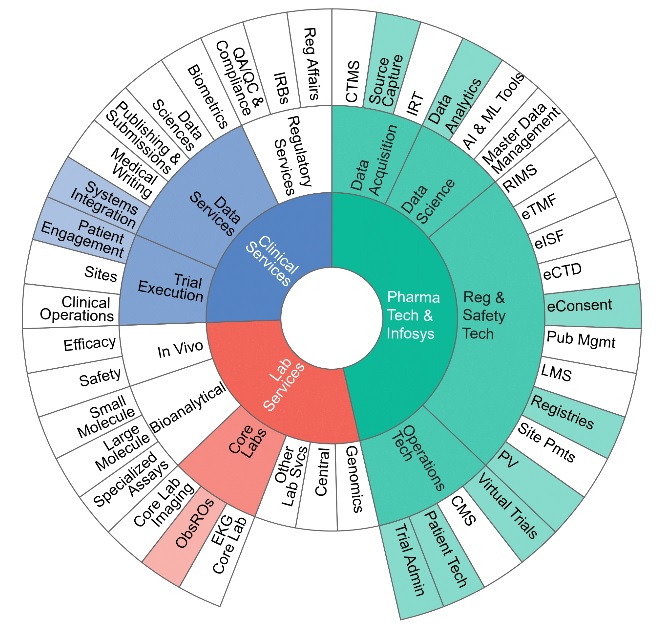
Decentralized study approaches make clinical research studies more convenient, engaging, data-driven and efficient.” — John Reites, President
Statistics:
Year Founded: 2015














