The Puerto Rico Consortium for Clinical Investigation (PRCCI) is the largest network of top academic and private research sites working in strong partnership with the wider Puerto Rico clinical investigation ecosystem to promote the island’s positioning as a preferred location for clinical investigation. PRCCI is a 501c3 non-profit organization established in April 2016.

Vision
Become the preferred partner for the implementation of clinical trials in minority groups.
Mission
To promote and expand clinical research activities in Puerto Rico:
• Optimizing the quality and speed of execution
• Establishing best practices to conduct clinical trials
• Building capacity and creating new job opportunities
• Educating patients, sponsors and researchers
These combined efforts result in a direct and indirect impact in inclusion of minority populations in clinical trials.

PRCCI enhances clinical research speed and quality by driving performance and efficiencies across our sites, leveraging strategic partnerships, increasing the skills and knowledge of professionals working through educational activities and establishing world-class capabilities in areas such as technology (e.g. Telemedicine tools ).
About Our Sites
• Highly qualified investigators with Board Certifications
• Quality Validated Sites by our external quality partner, Yale Center for Clinical Investigation (YCCI)
• Resilient sites with solid experience equipped to continue operations during natural disasters
• Access to a minority population exposed to participating in clinical research trials
• Phase I-IV clinical trial capabilities
PRCCI’s member sites have experience in a wide and diverse range of therapeutic areas. These areas include, but are not limited to the following:


For Patients
Offering patients access to innovative treatments through clinical trials
We are focused on promoting Puerto Rico-based patients’ well-being by directly collaborating with patient advocacy groups and expanding our educational initiatives related to clinical investigation and diseases. The targeted segments are both patients and the broader population who can contribute through PRCCI to advancing science, facilitating access to new innovative treatments, and helping others.
For Sponsor and CROs
Improving the speed and quality of your clinical trials
Working with PRCCI provides one-stop access to an experienced network of investigators, fast site selection process, access to a large population of minority patients in multiple therapeutic areas, a single point of contact for quick and efficient study start-up and, ongoing quality monitoring of our consortium members. Timely and effective implementation of recruitment and retention strategies.

For Research Sites
Joining the PRCCI network will help you increase the access to clinical trials in diverse therapeutics areas. We work on your behalf to promote your site, identify and secure research opportunities, conduct budget and contract negotiations, monitor and improve your quality processes, and more.
Proud to be part of PR’s Clinical Investigation Ecosystem
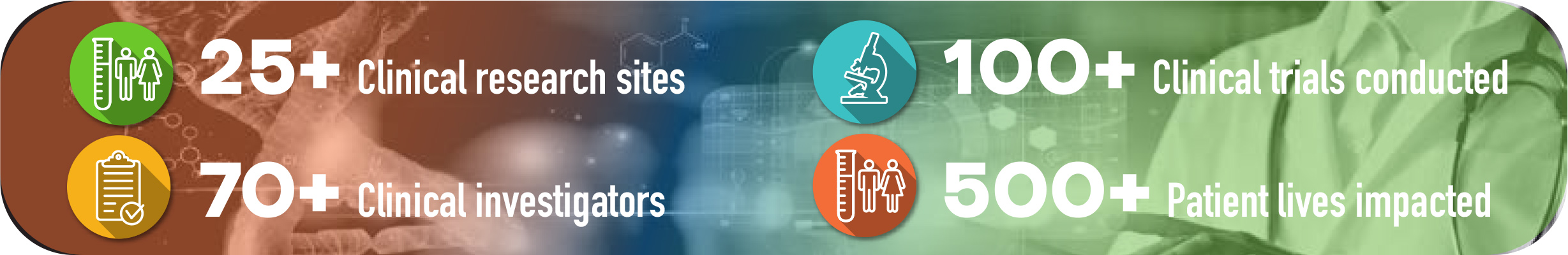
We are proud to be part of this talented and committed group of professionals that along with PRCCI generated a steady performance during the 2016-2020 period. Results attest the ecosystem’s resiliency in coping with natural disasters that have impacted PR ranging from hurricanes, such as Irma and María, earthquakes on the Island’s southern region, to the COVID-19 pandemic- all occurring during this time frame.
2016 – 2020 Puerto Rico Clinical Investigation Activity
(Source: www.clinicaltrials.gov)
A significant growth has been achieved between 2016 and 2020: 536 clinical trials were conducted during this period.

We at PRCCI look forward to the opportunity of partnering with you and your company to facilitate all the processes necessary for the execution of a successful clinical investigation trial.













