With over 55,000 active clinical trials today, patient recruitment is the greatest challenge the industry faces to successfully completing studies on time – sponsors spend large amounts to onboard sites, the sites often fail to meet enrollment targets, and studies often become delayed significantly. Historically, pharma has only relied on research sites to identify and enroll patients in clinical trials, with no direct-to-patient outreach.
This model may have worked when the major focus was on chronic conditions and the population sizes were in the millions. However, in the past 20 years and more recently with the help of tech advancements, the pharmaceutical industry shifted focus on rare disease and oncology, which account for nearly 40% of active trials today. In the rare disease and oncology space, where patient populations are extremely small and dispersed across the globe, sites fail to reach enrollment goals nearly 80% of the time, exaggerating the sponsor’s cost to run trials.
Pharmaceutical and biotech companies need to engage with patients directly and not rely exclusively on sites to recruit patients, but no industry-wide platform exists that enables sponsors to communicate directly with patients before, during or after clinical trials. We launched PatientWing, a platform that empowers Sponsors to do exactly that – engage with patients, to learn from them and create more patient-centric trials, to enroll patients in their trials, to advance science and save lives.
Just a Quick Note:
InnovationsOfTheWorld.com has partnered with Trade License Zone (TLZ) to support global innovators looking to expand internationally. Take advantage of the UAE’s Free Zones—enjoy streamlined setup, low corporate taxes, and a strategic gateway to the Middle East and beyond.
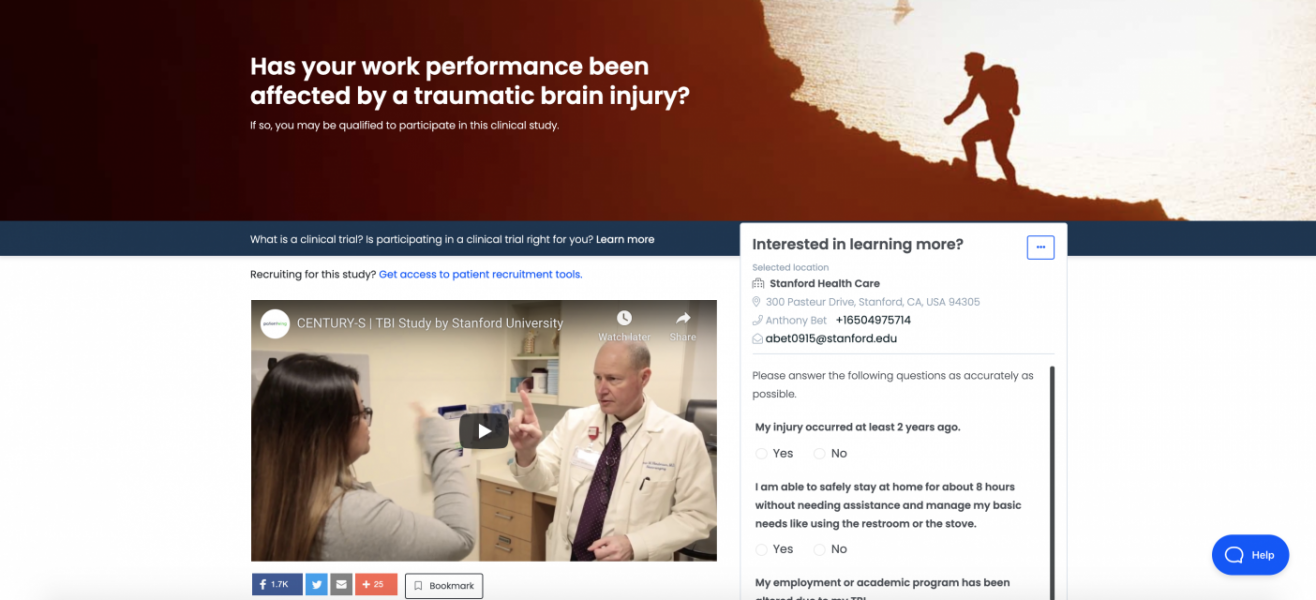
Get Your UAE Free Zone License Fast & Easy!At the surface, our website is a patient-friendly interface where you can search for active clinical trials, synced nightly with clinicaltrials.gov . For sponsors, we offer two main solutions: Enroll., a patient recruitment solution that meets patients where they are to exceed enrollment goals, and Engage., a fully branded, end-to-end solution that enables that communication before, during and after clinical trials.
Our platform is GDPR, HIPAA and 21 CFR Part 11 compliant and is designed to give control back to sponsors. With industry regulations and strict patient privacy laws, it has been a common misconception that sponsors are unable to interact with their patients, let alone the recruitment process.
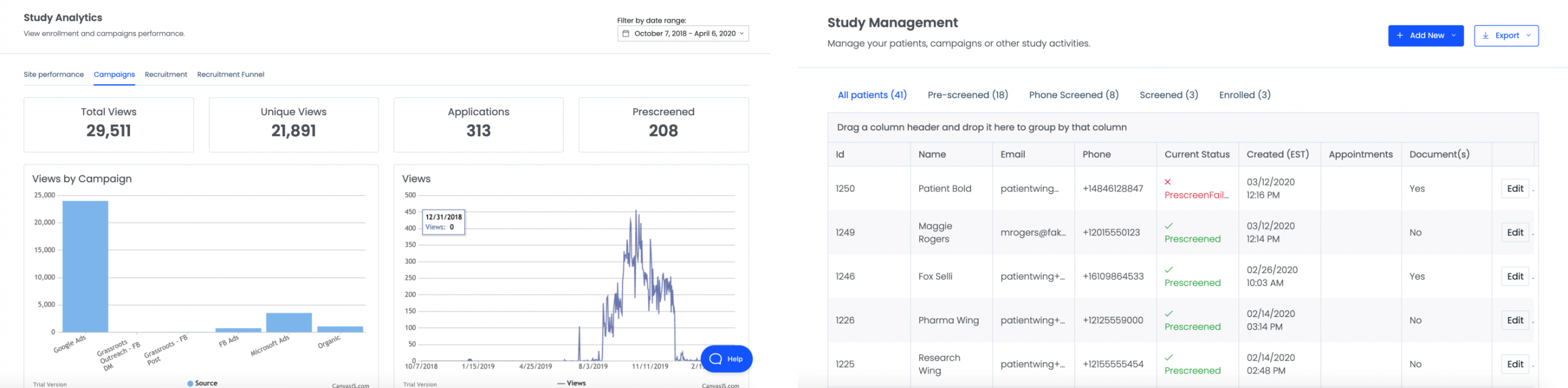
By anonymizing patient data for the sponsor, we allow sponsors to review enrollment metrics in real time and make necessary changes to the protocol, marketing spend, or even site locations to improve enrollments and complete trials on time.
Clinical trials are a dynamic process with millions of dollars and thousands of lives on the line. Sponsors need to feel empowered to engage with patients but lacked the appropriate tools to date. We’re here to change that.
With our platform, we connect patients with the correct clinical trials and enable sponsors to engage patients before, during and after clinical trials.
Statistics:
Year Founded: 2016
Founded By: Zikria Syed – CEO, Paul Elisii – COO and Todd Keuny – CTO














