Selective Targeting of GTPases – A novel, promising approach to treating Cancer, Autoimmune Conditions and Chronic Fibrotic Disease
MBQ PHARMA – The Company and its mission
MBQ Pharma is a clinical-stage pharmaceutical company dedicated to developing treatments with high unmet medical needs such as Cancer, Autoimmune Disease and Chronic Fibrotic Syndromes.
Our initial program has focused on developing anti-cancer agents capable of improving overall survival. Our lead compound MBQ-167 is a small molecule that markedly inhibits tumor growth and metastasis. Our compounds selectively target the small GTPases Rac1 and Cdc42, which are central drivers of processes leading to cancer cell proliferation, tissue invasion, angiogenesis and metastasis formation. MBQ-167 abolishes cancer cell motility, growth, and survival, essential elements of cancerous growth. Strong published and unpublished data show that MBQ-167 targets metastasis by blocking cancer cells from leaving the primary tumor, impeding the establishment of new metastases, and reducing existing metastases. Compared to other approaches targeting Rac and Cdc42, MBQ-167 has an unparalleled degree of specificity, affinity, and safety, and most importantly, its efficacy is not affected by mutations of the targeted molecules.
Our first indication for our lead compound MBQ-167 is the treatment of patients with advanced breast cancer, including those with metastasis. Emphasis will be on treating breast cancer with high unmet medical needs, such as triple-negative breast cancer (TNBC) and metastatic cancer. Later programs will include studies on disease recurrence prevention, for example, for those patients with existing bone marrow micrometastases and/or circulating tumor cells. MBQ-167 holds great promise for treating CNS tumors because its chemical properties indicate the ability to cross the blood-brain barrier. Moreover, our non-clinical data show that MBQ167 has a strong potential to be effective in treating metastatic disease caused by other solid tumors including pancreas, lung, and brain cancer.
In June 2022, FDA granted MBQ Pharma an IND for MBQ-167 to be tested in its First-in-Human Trial in patients with advanced breast cancer. The phase 1 safety clinical trial will start during the summer 2023.
Breast Cancer Mortality due to Metastasis and disease Recurrence – A high unmet medical need
While the five-year survival rate for localized breast cancer is about 99%, the longer-term survival has not improved significantly during the past decades. A sizable portion of all breast cancer patients who have survived five years will have recurrent disease and metastasis that eventually lead to premature death. Accordingly, an estimated 90% of breast cancer deaths result from metastatic disease, whether the cancer was metastatic at diagnosis or a metastatic recurrence developed later. Therefore, the survival rate for advanced metastatic breast cancer remains at only 22%, with a median survival of four years. Additionally, an estimated 20% to 30% of women diagnosed with invasive breast cancer will have a recurrence and may eventually die. Therefore, beyond finding an effective treatment for advanced breast cancer, the key challenges to improving overall survival remain the prevention and treatment of disease recurrence, which typically presents itself with an aggressive course and widespread metastasis. Even though the introduction of targeted and/or immunomodulating therapies such as PD-1/PD-L1 inhibitors have shown some benefit to date, they have not significantly impacted overall survival. Moreover, targeted therapies often face the development of tumor resistance, and immunomodulating approaches come at very high costs and frequent severe toxicities, often further compromising the quality of life for patients. So far, no effective standard treatment for triple-negative and inflammatory breast cancer has been established.
Moreover, many studies have shown that disease recurrence and shortened survival are linked to micrometastasis in the bone marrow or circulating tumor cells in the blood at the time of diagnosis, even in patients with localized disease. Thus, micrometastases and circulating tumor cells significantly increase local and distant disease recurrence risk and shortened survival. Until today, no treatment can prevent recurrence or successfully treat metastatic disease.
Taken together, new therapeutic and prognostic diagnostic strategies for breast cancer are needed to improve survival outcomes for breast cancer patients. Moreover, the absence of curative treatment regimens for advanced and metastatic disease at diagnosis or recurrence further highlights the high unmet medical needs in breast cancer treatment. MBQ Pharma’s clinical development strategy through lead compound MBQ-167 meets this unmet medical need. Our initial data suggest that based on the mechanism of action of MBQ-167 paired with our positive preclinical data in other tumor types suggest that our lead compound will also show efficacy in many other tumors.
Our lead compound MBQ-167 eradicates breast cancer metastases in various animal models and prevents metastasis formation. In addition, MBQ-167 has notably revealed an excellent safety profile in FDAmandated toxicity analyses in rodents and dogs. It thus qualifies the compound to be used as possibly longer term and in combination with established chemotherapeutic and targeted treatments. In fact, in several TNBC models, MBQ-167, combined with Paclitaxel, the current standard of care for TNBC, showed a strongly improved and synergistic effect in preventing and removing pre-existing lung metastases.
In addition to the observed effects of MBQ-167 specifically targeting metastasis, our data with stem cell-like breast cancer models show that MBQ-167 may have a beneficial effect in treating micrometastasis and, most importantly, stop the reactivation of putative dormant cancer cells in the body. If this hypothesis proves correct, MBQ-167 could potentially be essential in preventing and/or treating cancer recurrence and metastasis.
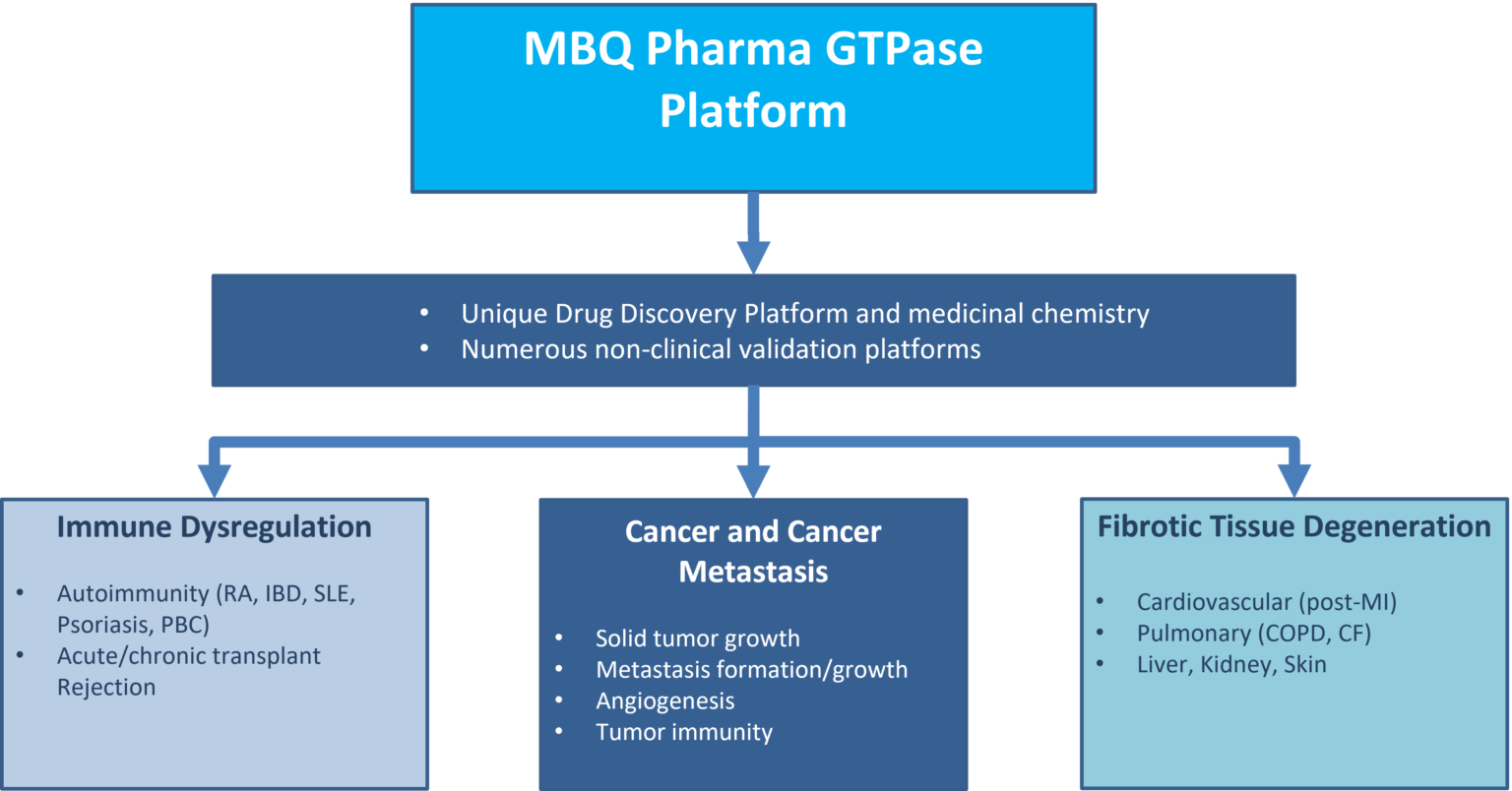
In addition to MBQ-167, MBQ-Pharma has a strong pipeline of related compounds differentially targeting GTPases. Its unique discovery platform can identify numerous highly effective high-affinity GTPase targeted molecules with differential effects on members of the small GTPase families and as well as proteins regulating GTPase activity (GEFs).
Our pipeline of compounds holds excellent promise as a new treatment paradigm not only for cancer but also other chronic diseases including autoimmunity and chronic fibrosis as they are pathogenetically linked to increased activity/expression of GTPases and related factors like GEFs.
The hitherto observed outstanding safety profile qualifies MBQ-167 as a promising approach to address the high unmet medical needs in cancer, autoimmunity and chronic fibrosis. . Moreover, data show that MBQ-167 in combination with other targeted therapies can overcome resistance to the latter and/or augment therapeutic benefits of immune therapies.












