A faster way of identifying, visualizing, managing, and documenting trial risks that could compromise patient safety and delay the approval of Investigational Products.
The clinical research industry is adopting Risk-Based Quality Management (RBQM) and Risk-Based Monitoring (RBM) as primary methods for managing quality. Formally endorsed by both the Food and Drug Administration (FDA) and European Medicines Agency (EMA), and now encoded in the ICH E6 (R2) GCP update, RBQM and RBM encourage relatively less reliance on traditional on-site monitoring practices including frequent site visits and 100% source data verification/review (SDV/SDR).
It also encourages greater reliance on centralized monitoring including the use of statistical methods to identify quality-related risks (e.g. unusual or unexpected data patterns). There is a growing body of evidence supporting both the importance of Centralized Statistical Monitoring (CSM) and the relatively low value of comprehensive SDV and SDR.
CluePoints Inc. is a leading provider of software solutions to enable and support RBQM, which combines a risk planning module (Risk Assessment & Categorization Tool) and a centralized monitoring and analytics platform CluePoints Central Monitoring Platform). CluePoints’ innovative software provides Sponsors and CROs with a better and faster way of detecting and managing risks that may impact the outcome of clinical trials.
Just a Quick Note:
InnovationsOfTheWorld.com has partnered with Trade License Zone (TLZ) to support global innovators looking to expand internationally. Take advantage of the UAE’s Free Zones—enjoy streamlined setup, low corporate taxes, and a strategic gateway to the Middle East and beyond.
Get Your UAE Free Zone License Fast & Easy!The solutions, all of which are cloud-based and enhanced with machine learning techniques, are driven by CSM, a unique set of algorithms that interrogate clinical and operational data in real-time centrally to conveniently illuminate outliers and anomalies in data. The adoption of statistics to assist with quality oversight in trials is well documented. So much so, that the FDA extended its relationship with CluePoints to further explore a data-driven approach to oversight in trials.
The following features support effective quality oversight and detection of operational study risk:
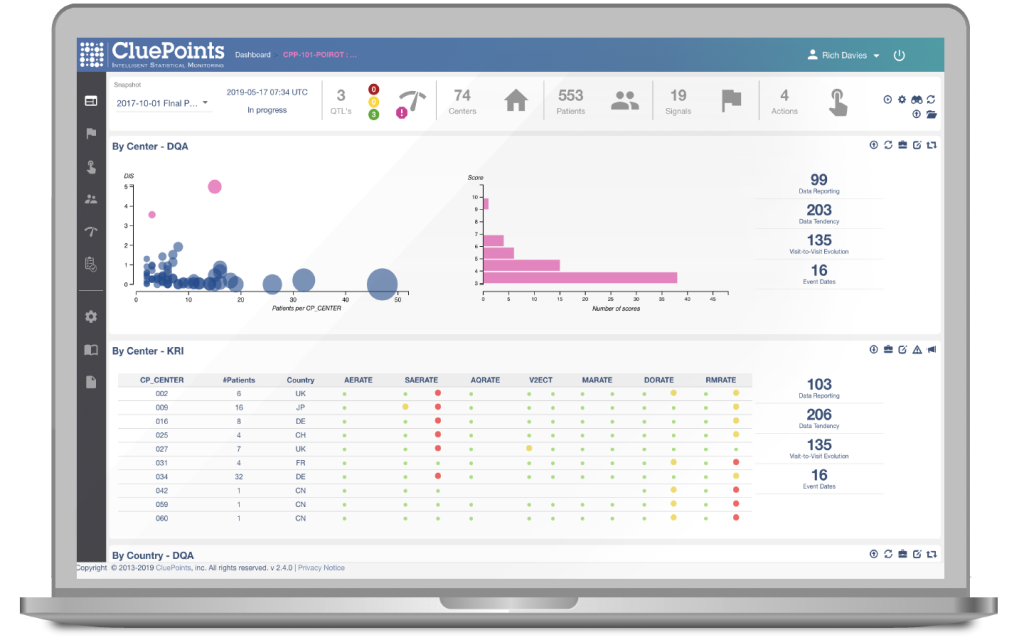
- Data Quality Assessment (DQA) – An advanced suite of statistical tests is executed across all clinical study data to identify unusual data patterns at sites (or regions, patients) representing potential issues.
- Key Risk Indicators (KRIs) – Any number of standard and study-specific KRIs can be configured to help identify specific risks of interest across sites (or regions, patients).
- Quality Tolerance Limits (QTLs) – Any number of QTL parameters can be configured to help identify study-wide issues that may impactful to overall study outcomes or patient safety.
- Patient Profiles – Patient profile reports can be configured for each study and used either to support investigation of identified risks or for medical/safety reviews.
- Duplicate Patients – This configurable module enables the detection of potential duplicate or “professional” patients.
- Data Visualizations – An advanced data visualization and reporting engine provides for both pre-configured and real-time dashboards and data exploration support.
CluePoints is the premier provider of Risk-Based Study Execution (RBx) and Data Quality Oversight Software. Our products utilize comprehensive statistical algorithms to determine the quality, accuracy, and integrity of clinical trial data both during and after study conduct. Aligned with guidance from the FDA, EMA, and ICH E6 (R2), CluePoints® is deployed to support central and on-site monitoring, medical review, quality risk management and to drive a holistic Risk-Based strategy in all trials.
Coupled with thought leadership and consulting expertise to aid pre-study risk assessment, identification of risk controls and solution implementation you now have everything you need to adhere with global regulatory guidance. The result is increased operational efficiency, lower costs and reduced regulatory submission risk as part of the industry paradigm shift to RBx.
Statistics:
Year Founded: 2012
Founded By: Marc Buyse, Francois Torche and Patrick Hughes













