OUR PURPOSE
4C Pharma is a comprehensive healthcare solutions company. Owned and operated by Medical Doctors with hands-on expertise in pharmacovigilance (drug safety) and regulatory domains, 4C’s core values of transparency, honesty, ownership, success, and enthusiasm allow it to provide high quality solutions that are compliant with current regulations while anticipating and preparing for future ones. Through this approach, 4C Pharma becomes a trusted partner and extension of any company it works for.

OUR FOCUS
Just a Quick Note:
InnovationsOfTheWorld.com has partnered with Trade License Zone (TLZ) to support global innovators looking to expand internationally. Take advantage of the UAE’s Free Zones—enjoy streamlined setup, low corporate taxes, and a strategic gateway to the Middle East and beyond.
Get Your UAE Free Zone License Fast & Easy!4C Pharma Solutions is a Service Provider Organization with a key focus on delivering service excellence to our partners through years of experience and expertise in the industry. Headquartered in New Jersey, with offices across major locations throughout the globe, they are well versed with FDA, EMA and other regulated as well as emerging markets. 4C is strategically located in the US, Europe, UK, UAE, and India for ease of doing business and allowing for an extensive global reach. The company follows a unique approach, where each solution is customized according to the partner. This reduces time and effort on the part of the end user, allotting it toward further research and development. 4C Pharma Solutions is a leader in pharmacovigilance with services including medical information call centers, case processing (from beginning to end), literature search, electronic submissions, periodic reporting, signal detection, risk management, hosting solutions, medical writing, CDM, QMS, and Regulatory Affairs.
OUR HISTORY
4C Pharma Solutions was established in 2014 by medical doctors who have practiced medicine in the past. Each individual brings a unique and overlapping complementary expertise from the fields of pharmacovigilance, clinical research operations, medical affairs, clinical medicine, regulatory, and technology implementations. This allows for an in-depth knowledge of drug and device development along with the requirements for maintenance. The aim for the company has been to work with small and large companies alike. With the intent to reduce cost, increase quality, and be compliant, 4C hopes a greater pool of companies bring new techniques to the industry to help a larger patient population.
OUR HOPES
4C Pharma solutions believes in not only helping their partners but also giving back to the industry and to the educational community. It is the responsibility that comes with success. As a company that offers training for industry experts and new comers alike, 4C wanted to take it the next level. For that reason, they have partnered with Temple University to further its Scholl of Pharmacy’s advancement of the school of pharmacy. 4C has also worked with the University’s RAQA program. For 50 years, Temple’s School of Pharmacy has been the foremost graduate program of its kind in the nation. Temple’s MS in RAQA transforms graduate education for pharmaceutical professionals. Being a strategic partner, and having invested in the continuing education of students, 4C has been a proud supporter of Temple University’s School of Pharmacy. As this partnership continues, the hopes are that the University and 4C can bring a new dimension to an industry that always needs trained individuals at the helm.

Founded in 1968, the Regulatory Affairs and Quality Assurance (RAQA) program’s accomplishments are impressive.
The program is the oldest, largest, and most comprehensive of its kind in the country. The University has more than 80 courses in Quality and Regulatory topics, and more than 300 students from the US, Canada, and South America take courses during its fall, spring, and summer semesters. Its faculty consists of nearly 100 industry and FDA professionals from across the country who have at least 15 years of experience in their fields. More than 2,500 students have received the MS in RAQA since the program’s inception.
RAQA’s mission is to provide excellent, affordable, higher education which prepares students for successful careers in the regulated healthcare industry or FDA.
The program has regular meetings with industry and FDA leaders to ensure that courses address the latest trends and practices. Thirteen excellent certificate programs are available. The program offers other industry-focused degrees, including the MS in Pharmaceutics and the MS in Global and Clinical Pharmacovigilance Regulations (GCPR). The latter was the first of its kind in the US and continues to accelerate careers paths in safety surveillance activities. RAQA’s relationship with 4C Pharma provides another layer of hands-on experience for students studying global pharmacovigilance.

RAQA has pioneered many student-friendly services at the graduate level.
Online courses are live transmissions. The dynamic online classrooms feature “learning by doing” and networking. Students engage in interactive discussions and problem-solving workshops. Courses accommodate professionals working full-time in industry who may only be able to pursue graduate studies on a part-time basis. With flexible entry procedures, students can delay applying to the MS while easing into graduate studies by first starting courses towards a certificate. After earning a certificate, students may apply for and complete the MS program or take up to five years off before returning to finish the MS degree.
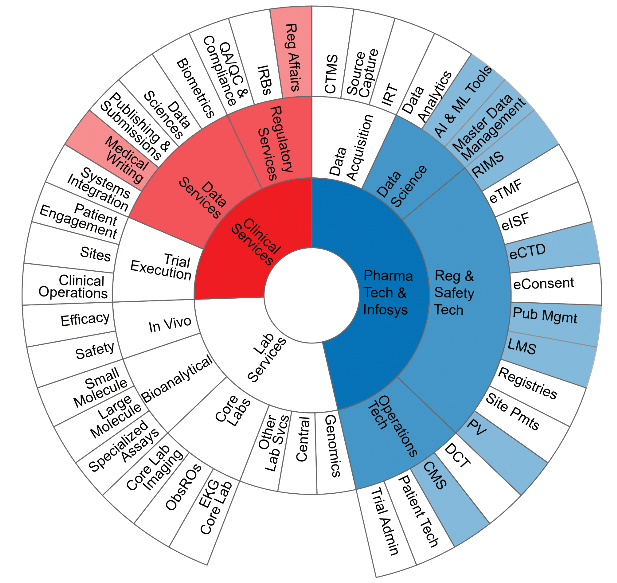
4C Pharma is a comprehensive healthcare solutions company. Owned and operated by Medical Doctors with hands-on expertise in pharmacovigilance (drug safety) and regulatory domains, 4C’s core values of transparency, honesty, ownership, success, and enthusiasm allow it to provide high quality solutions that are compliant with current regulations while anticipating and preparing for future ones. Through this approach, 4C Pharma becomes a trusted partner and extension of any company it works for.
Statistics:
Year Founded: 2014
Founded By:
Muhammad Ahmad, MD MBA and Yathendra Madineni, MD













