Arcturus is a leading clinical-stage messenger RNA medicines company focused on the discovery, development and commercialization of therapeutics for rare diseases and vaccines.

Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a clinical-stage mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR™ mRNA Technology and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus’ diverse pipeline of RNA therapeutic candidates includes programs to potentially treat Ornithine Transcarbamylase (OTC) Deficiency, Cystic Fibrosis, Glycogen Storage Disease Type 3, Hepatitis B, non-alcoholic steatohepatitis (NASH) and a self-replicating mRNA vaccine for SARS-CoV-2. Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, replicon RNA, antisense RNA, microRNA, DNA, and gene editing therapeutics. Arcturus’ technologies are covered by its extensive patent portfolio (187 patents and patent applications, issued in the U.S., Europe, Japan, China and other countries). Arcturus’ commitment to the development of novel RNA therapeutics has led to collaborations with Janssen Pharmaceuticals, Inc., part of the Janssen Pharmaceutical Companies of Johnson & Johnson, Ultragenyx Pharmaceutical, Inc., Takeda Pharmaceutical Company Limited, CureVac AG, Synthetic Genomics Inc., Duke-NUS, and the Cystic Fibrosis Foundation. For more information visit www.ArcturusRx.com
About Coronavirus
Coronaviruses are a family of viruses that can lead to respiratory illness, including Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). Coronaviruses are transmitted between animals and people and can evolve into strains not previously identified in humans. On January 7, 2020, a novel coronavirus (2019-nCoV) was identified as the cause of pneumonia cases in Wuhan City, Hubei Province of China, and additional cases have been found in a growing number of countries.
About Arcturus’ COVID-19 vaccine candidate
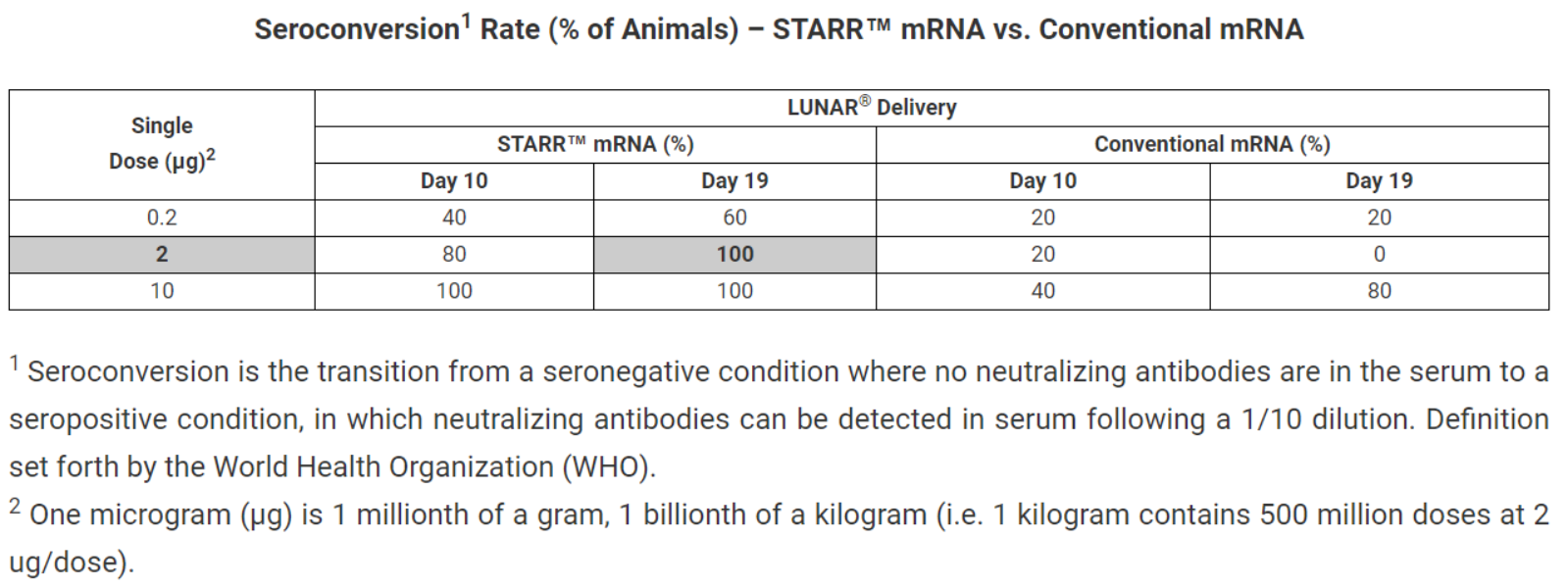
Arcturus announced positive immunogenicity data from its preclinical study for the Company’s COVID-19 Vaccine. Seroconversion, using a gold standard virus neutralization assay (Vero-E6 cells with a SARS-CoV-2 Singapore Clinical Isolate), and IgG/IgM antibody titers were assessed at day 10 and day 19. Rodents were immunized with a single dose (0.2, 2, and 10 µg, i.m.) of LUNAR-COV19 vaccine. The study results showed self-transcribing and replicating (STARR™) mRNA induced higher seroconversion relative to conventional mRNA at equivalent doses. In conjunction anti-SARS-CoV-2 IgG and IgM antibody titers were also higher. GMP-Manufactured batch to be delivered in June 2020 and the human dosing is expected to begin in summer 2020.
Just a Quick Note:
InnovationsOfTheWorld.com has partnered with Trade License Zone (TLZ) to support global innovators looking to expand internationally. Take advantage of the UAE’s Free Zones—enjoy streamlined setup, low corporate taxes, and a strategic gateway to the Middle East and beyond.
Get Your UAE Free Zone License Fast & Easy!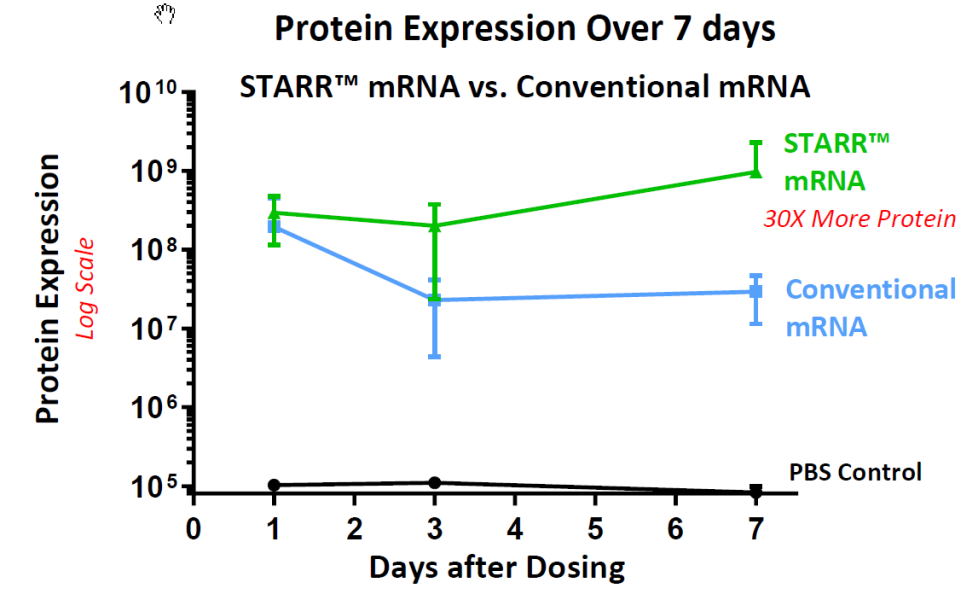
About STARR™ Technology
The STARR™ Technology platform combines self-replicating RNA with LUNAR®, a leading nanoparticle delivery system, into a single solution to produce proteins inside the human body. The versatility of the STARR™ Technology affords its ability upon delivery into the cell to generate a protective immune response or drive therapeutic protein expression to potentially prevent against or treat a variety of diseases. The self-replicating RNA-based therapeutic vaccine triggers rapid and prolonged antigen expression within host cells resulting in protective immunity against infectious pathogens. This combination of the LUNAR® and STARR™ technology is expected to provide lower dose requirements due to superior immune response, sustained protein expression compared to non-self-replicating RNA-based vaccines and potentially enable us to produce vaccines more quickly and simply.
FDA Allowance and CTA Approval
Arcturus announced the acceptance of two clinical trials for its flagship asset ARCT-810, also known as LUNAR-OTC, a first-in-class mRNA therapeutic being developed to treat ornithine transcarbamylase (OTC) deficiency. The Company’s Investigational New Drug (IND) application for a Phase 1b study in patients with OTC deficiency was allowed to proceed by the U.S. Food and Drug Administration (FDA), and an additional Clinical Trial Application (CTA) for a Phase 1 study in healthy volunteers was approved by the New Zealand Medicines and Medical Devices Safety Authority (Medsafe).

About ARCT-810
ARCT-810, Arcturus’ first development candidate, represents a novel approach to treat ornithine transcarbamylase deficiency. ARCT-810 is based on Arcturus’ mRNA design construct and proprietary manufacturing process. ARCT-810 also utilizes Arcturus’ extensive and propriety lipid library and employs the Company’s LUNAR® delivery platform to deliver OTC mRNA to hepatocytes. ARCT-810 is an investigational mRNA medicine designed to enable OTC-deficient patients to naturally produce healthy functional OTC enzyme in their own liver cells. Replacing the deficient OTC protein has the potential to restore activity of the urea cycle pathway, resulting in reduced plasma ammonia and urinary orotate concentrations.














